Julien publishes his paper in Chem. Mater!
Insights in SSZ-13 Al distributions from (IZC) synthesis have been unraveled...
... handing us the levers to optimize redox catalysis by bottom up design (e.g. for methane partial oxidation)
High Si SSZ-13 synthesis is tuned towards optimal divalent cation capacity (DCC). The effectiveness of the applied synthesis strategies are demonstrated by methane partial oxidation (MPO) tests with record high (above statistical) methanol productivity from post-synthesis exchange. The uncovered (synthesis) relations uncovered in this work can inspire the search for catalytically relevant Al-ensembles in zeolites used in a diverse range of transition-metal ion based reactions.
This work is the result of fruitful cooperation with the Sels group and an interdepartemental collaboration with Dr. Cedric Van Goethem and Prof. Jin Won Seo, and includes a contribution from Dr. Son-Jong Hwang from Caltech (California, USA).
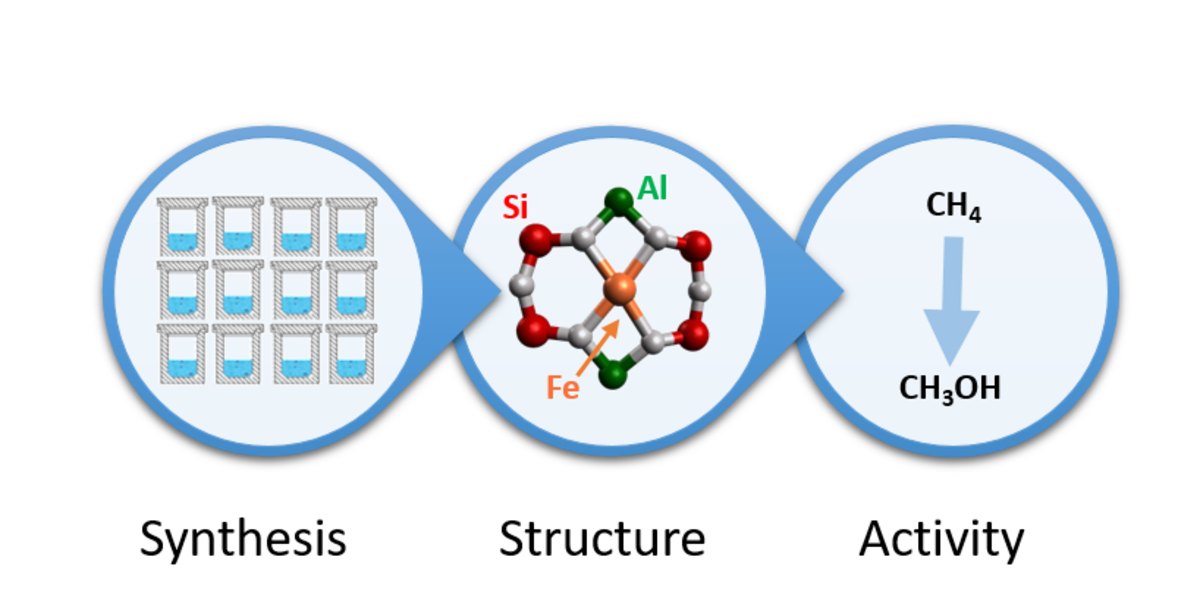
[50] Synthesis-structure-activity relations in Fe-CHA for C-H activation: control of Al-distribution by interzeolite conversion
![[50] Synthesis-structure-activity relations in Fe-CHA for C-H activation: control of Al-distribution by interzeolite conversion](https://dusselier-lab.org/uploads/media/cache/default/uploads/ee488d2d3d457a829c7759b95b1d8bcd.jpeg)
The search for structurally relevant Al-arrangements in zeolites is an important endeavor for single site catalysis. Little is known about the mechanisms and zeolite dynamics during synthesis that are responsible for creating those Al-ensembles. Here, new synthetic strategies for creating Al-hosts in small-pore zeolites suitable for divalent cation catalysis are uncovered, leading to a mechanistic proposal for Al-organization during crystallization. As such, unique synthesis-structure-activity relations are demonstrated for the partial oxidation of methane on Fe-exchanged CHA-zeolites. With modified interzeolite conversions, the divalent cation capacity of the resulting high Si SSZ-13 zeolites (Si/Al ~ 35) can be reproducibly controlled in a range between 0.04 and 0.34 Co2+/Al. This capacity is a proxy for the distribution of framework aluminum in pairs and correlates with the methanol production per Al when these zeolites host the α-FeII redox active site. The uncovered IZC synthesis-structure relations paint an Al-distribution hypothesis, where incongruent dissolution of the starting USY zeolite and fast synthesis kinetics with atypical growth modes allow assembling specific Al-arrangements, resulting in a high divalent cation capacity. Prolonged synthesis times and high temperatures overcome the energetic barriers for T-atom reshuffling favoring Al-isolation. These mechanisms and the relations uncovered in this work will guide the search for relevant Al-ensembles in a range of zeolite catalysts where controlling the environment for a single active site is crucial.